Research & Development
The efficacy and safety of Nocturol were studied in a Phase 2 double-blinded clinical trial in New York City and Long Island, NY. The underlying science was rock solid so the stunning results of the trial were not a surprise.
86 patients were divided into 4 groups, with 21 taking 3 placebo pills, 22 taking 3 Nocturol pills, 21 taking 1 placebo and 2 Nocturol pills, and 22 taking 2 placebos and 1 Nocturol pill. After completing an initial physical, the qualified patients kept a nocturia diary for 2 weeks to establish a baseline of their nocturia frequency. Upon returning to the clinic, patients filled out a nocturia quality of life survey to establish a baseline reflecting their happiness during the prior 2 weeks. They returned their diaries and were then given new diaries and a 2-week supply of pills. The pills were to be taken 30 minutes before bedtime to allow the drug to begin its work before the bladder is emptied one last time before getting into bed. Upon completing the second 2-week period, the quality of life survey was once again completed and the diaries are collected.
As with any clinical trial, monitoring drug safety was a high priority, but based on the safety of the underlying drugs when taken in low doses, we expected and had no safety issues. The other major purpose of the trial was to evaluate the efficacy of various doses of Nocturol in reducing middle of the night bathroom visits.
A prior study using mouse and human bladder cells conducted at Ohio State University demonstrated that efficacy would not change with higher doses, but again scientific logic needed to be supported with clinical trial results to be certain about proper dosing. As expected, all 3 doses had similar results.
The below published studies have documented the synergy between Acetaminophen and NSAIDs (like ibuprofen) in treating fever, pain, and other inflammations.

Pre-existent asymmetry in the human cyclooxygenase-2 sequence homodimer.

Synergism between paracetamol (also known as acetaminophen) and nonsteroidal anti-inflammatory drugs in experimental acute pain.

New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases
Informal Studies and Phase 2 Details
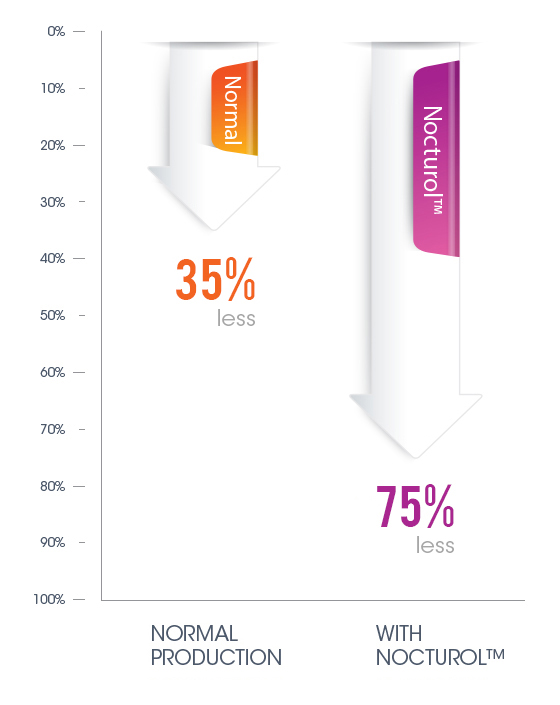
Informal studies showed the direct impact of Nocturol on prostaglandin production in the bladder. Whereas due to normal Circadian rhythm activity, prostaglandin production in the bladder tends to naturally decline about 35% overnight, when using Nocturol the reductions ranged from 71% to 75%.
By collecting consistently gathered urine samples for a few nights, this result could easily be verified by scientists or healthcare professionals for a few hundred dollars with little fear of placebo effects. It is unlikely that any patients could imagine their way to lowering prostaglandin production, so the easily observable reductions can only be caused by the combined effects of acetaminophen and ibuprofen.
The results of the 86 patient placebo controlled trial were backed up by a P value of 0.0017, which is far better than the 0.05 target normally used, so there is little doubt about the results. The results by patient varied widely as expected because while some patients’ nocturia is only caused by excess prostaglandin production, most patients are also affected by polyuria or the timing of their choices of foods and drinks that contain caffeine or alcohol. Even the time that patients go to bed can affect nocturia, since there is a Circadian rhythm associated with prostaglandin production. Going to bed at 8pm is probably the worst plan and going to bed at midnight is probably the best plan if you want to minimize nocturia events, since prostaglandin product increases dramatically each evening until midnight.
In our clinical trial, 23% of the patients reported a 50-96% reduction in nightly bathroom visits, while 38% reported a 25-50% reduction. Placebo patients reported a 10% reduction, which is far different than the results in other nocturia clinical trials, which sometimes have 50% nocturia reductions for placebo patients. This dramatic difference is because we instructed patients before they started their baseline data recording regarding the fact that what they drink and when they drink it can have significant effects upon their nocturia results.
The other positive factor for our future trials is that results are likely to improve because: a) patients had 5% better results in their second week of using our drug, and b) patients drank 2-18 ounces of water with their 3 pills (only 1 pill will be used in future trials), and c) only 21% of the patients were women while 52% of nocturia patients are women and women had results 3 percentage points better than the men. We did not share our lifestyle instructions with patients, but our expectation is that many of them will experience much better results if they follow the instructions carefully.
By providing access to Nocturol and proper education, our future licensees will be able to give most patients the ability to enjoy a dramatic improvement in their quality of life and overall health.
An article describing our Phase 2 clinical trial results was published in Neurourology and Urodynamics, the journal of the International Continence Society. You may read it by clicking on this link: https://onlinelibrary.wiley.com/doi/10.1002/nau.23910. One of the authors of the article was Dr. Jeffrey Weiss, who is perhaps the world’s leading authority on nocturia. He was the first author listed on the book Nocturia, Causes, Consequences, and Clinical Approaches. Yet another source of information regarding our drug is the new book recently published by Dr. King Lee and Dr. Jeffrey Weiss titled: Nocturia – Etiology, Pathology, Risk Factors, Treatment, and Emerging Therapies.